
What to know before you go (to see your doctor)
When you’re ready to give those pesky bumps the treatment they deserve, this guide will help get the conversation going.
Download the guide and ask your healthcare provider for YCANTH.
YCANTH is a prescription medication with an active ingredient called cantharidin, a blistering agent derived from a substance found in nature.* Although cantharidin has been used therapeutically for decades to treat other skin conditions, YCANTH is the first FDA-approved cantharidin treatment for molluscum contagiosum in adult and pediatric patients 2 years of age and older.
No other cantharidin compounds have met the strict requirements of the FDA for purity or safety.
According to the FDA: “Do not purchase or use nonprescription (over-the-counter, or OTC) products that claim to treat molluscum, even if the companies make statements that suggest their product may have been reviewed or is endorsed by the FDA. Some companies may mislead consumers by saying their product is ‘FDA-approved,’ is ‘FDA registered,’ is made in an ‘FDA-registered facility,’ or ’complies with FDA Current Good Manufacturing Practices’ (or ’CGMPs’).”

When you’re ready to give those pesky bumps the treatment they deserve, this guide will help get the conversation going.
Download the guide and ask your healthcare provider for YCANTH.
YCANTH can help start the healing process to clear the bumps in a matter of weeks.
During your in-office visit, your trained, licensed healthcare provider will apply a small, targeted amount of YCANTH directly to each bump using the specially designed applicator.
YCANTH usually causes small blisters on your skin to help remove the virus. Blistering is expected, with 98% of lesions smaller than a dime. Because you can see blisters forming, you can see YCANTH working.
In clinical trials, patients received between 1 and 4 treatments.
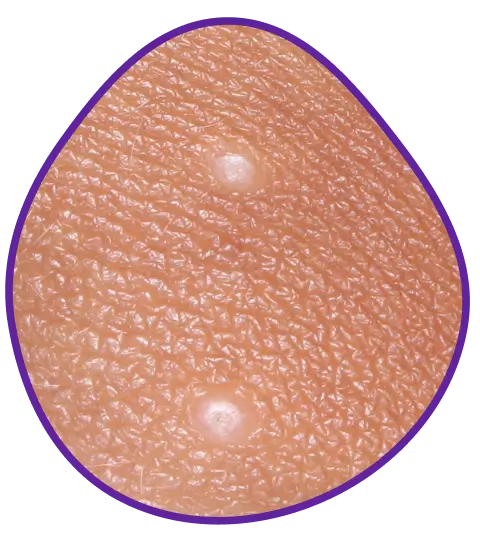
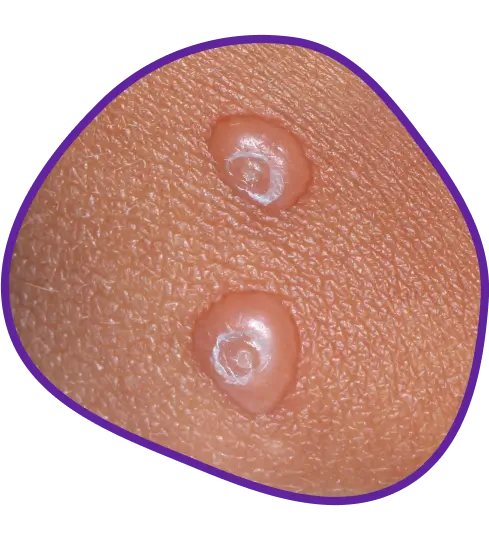

Before-and-after results of an actual patient throughout 12 weeks of treatment with YCANTH in a phase 3 clinical trial. Photos are not representative of all patients and individual results may vary. The most common side effects for YCANTH were local skin reactions at the application site, including blistering, pain, itching, scabbing, redness, and discoloration. These reactions are expected and are related to the anticipated blistering caused by YCANTH.
Two studies showed that YCANTH is an effective treatment for molluscum.
More than 500 people with molluscum were included in both studies.† Most were children aged 2 to 11 years, and about 10% were
†In clinical trials, 311 patients were treated with YCANTH, while 216 patients were given a placebo.





78% of people achieved 75% clearance or better by the end of their treatment with YCANTH compared with 35% of people treated with a placebo.
YCANTH can completely clear molluscum bumps with
Skin reactions are related to the way YCANTH works.
‡Based on phase 3 clinical trials.

Tips for care after treatment
Your trained, licensed healthcare provider will explain how to care for your bumps after you go home, but here are a few things to remember:
Download our After Treatment Guide for additional instructions, including Important Safety Information about YCANTH.
Be sure to schedule a follow-up appointment before you leave the office. You will want to share your progress and see if additional treatments are needed.
In clinical trials, the majority of application site reactions with YCANTH were mild to moderate.
The most common side effects for YCANTH were local skin reactions at the application site, including blistering, pain, itching, scabbing, redness, and discoloration. These reactions are expected and are related to the blistering caused by YCANTH.
For full Important Safety Information, click here.


With the YCANTH Copay Assistance Program, commercially insured patients can save on YCANTH.
§Program is solely for patients’ charges incurred in the use of YCANTH (cantharidin) topical solution and does not include any other related charges. The Program is only for qualified commercially insured patients seeking FDA approved treatments consistent with the YCANTH label. For all qualified patients, Verrica is responsible for all YCANTH product costs under the Program amount and excluding the copay requirement. The patient’s insurance provider can provide the most accurate explanation of all charges. Approval to the Program is not guaranteed. Program has a lifetime maximum benefit of $2,805 or 4 treatments for YCANTH, whichever occurs first. The copay program covers up to two (2) YCANTH applicators per visit depending on patient need. Until the patient reaches the maximum Program benefit, providing healthcare professionals may not charge the patient more than the applicable Program allowance. Patient will bear financial responsibility for all costs not covered by commercial insurance exceeding maximum benefit for YCANTH. THIS IS NOT INSURANCE. Not valid for prescriptions paid, in whole or in part, by Medicaid, Medicare, VA, DOD, TRICARE®️, or other federal or state programs including any state pharmaceutical assistance programs. This Program is not valid where prohibited by law, taxed or restricted. Verrica reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Additional terms and conditions may apply.
TRICARE® is a registered trademark of the Department of Defense (DOD), DHA.
Get the facts you need with summarized information about YCANTH sent to your inbox so that you can get up to speed fast.
YCANTH (cantharidin) topical solution, 0.7% is indicated for the topical treatment of molluscum contagiosum in adult and pediatric patients 2 years of age and older.
Do not get YCANTH in the mouth, nose, or eyes, and do not let YCANTH touch healthy skin. Life threatening or fatal toxicities can occur if YCANTH is taken by mouth. Avoid contact with areas of the body that have been treated, including contact by mouth. Damage to the eyes can occur if YCANTH comes in contact with the eyes. If YCANTH gets in the eyes, rinse eyes with water for at least 15 minutes.
Local skin side effects at the application site may occur, including blistering, itching, pain, discoloration, and redness. Do not get YCANTH in the mouth, nose, or eyes, and do not let YCANTH touch healthy skin. If YCANTH contacts any unintended surface or healthy skin, immediately remove. If severe blistering, severe pain, or other severe skin side effects occur, wash off YCANTH immediately and contact your healthcare provider.
YCANTH is flammable, even after drying. Do not expose YCANTH-treated areas to fire, flame, or smoke until YCANTH is washed off.
Do not use YCANTH if you are allergic to any of the ingredients in YCANTH. The active ingredient in YCANTH is cantharidin. The inactive ingredients are acetone, camphor, castor oil, denatonium benzoate, ethanol, gentian violet, hydroxypropyl cellulose, and nitrocellulose.
The most common side effects are local skin side effects at the application site, including blistering, pain, itching, scabbing, redness, discoloration, dryness, swelling, and loss of the outer layer of skin at the application site. These local skin side effects are expected and are related to the anticipated blistering caused by YCANTH.
It is not currently known if any medications interact with YCANTH.
The potential risk of YCANTH for major birth defects, miscarriage, or maternal or fetal side effects is unknown. Given that YCANTH is applied to the outside of the mother’s skin, use is not expected to result in exposure of YCANTH to unborn babies.
Do not use YCANTH on areas of the mother’s body where YCANTH may come in contact with the breastfeeding child’s mouth or eyes.
Taking cantharidin by mouth has resulted in kidney failure, blistering and severe damage to the stomach and intestines, excessive bleeding or clotting, seizures, and weakness or paralysis. Seek medical attention immediately if YCANTH is accidentally ingested.
These are not all the possible side effects of YCANTH. Call your doctor for medical advice about side effects. You are encouraged to report side effects to the FDA at
Please see full Prescribing Information.