INDICATION:
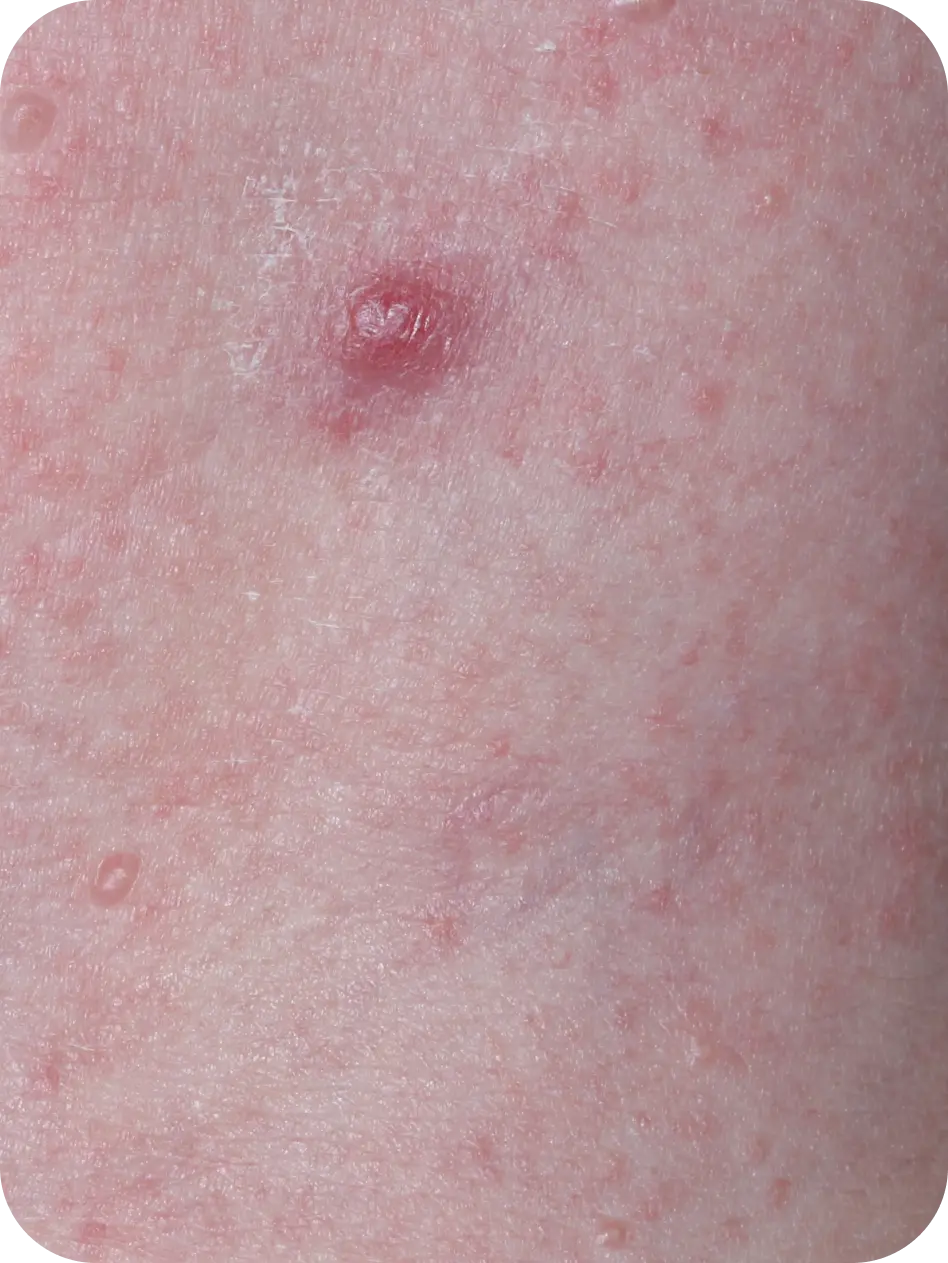
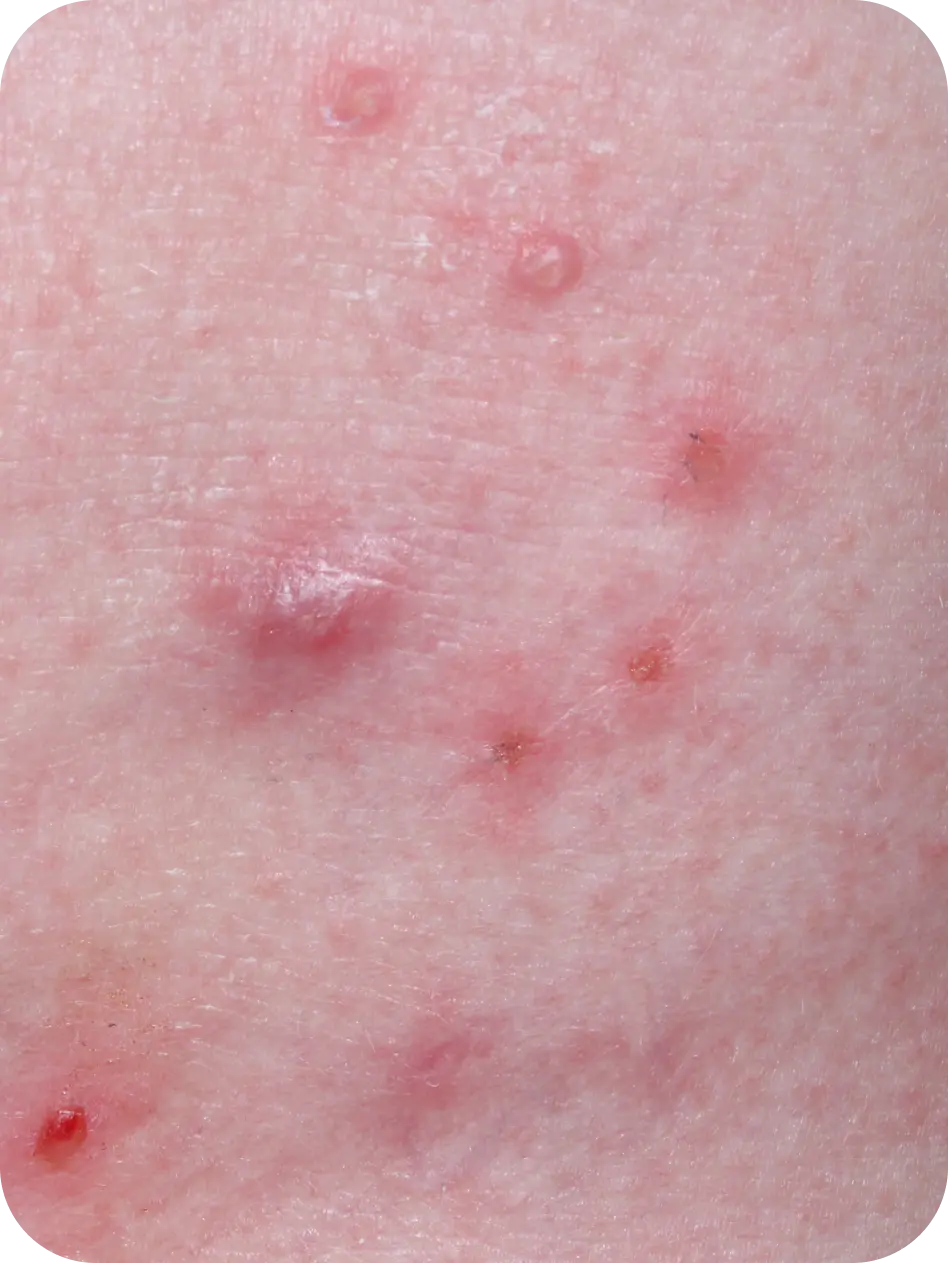
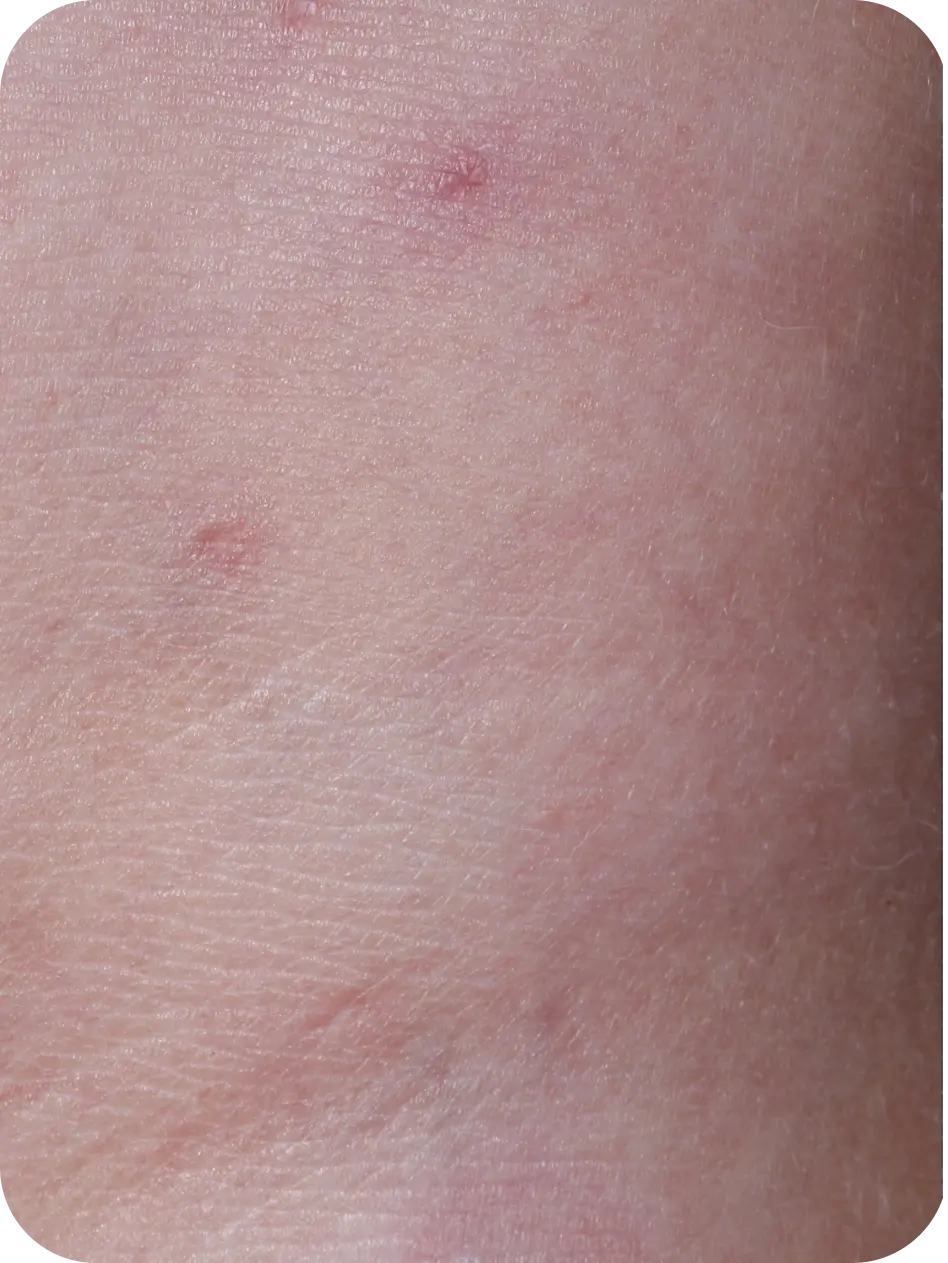
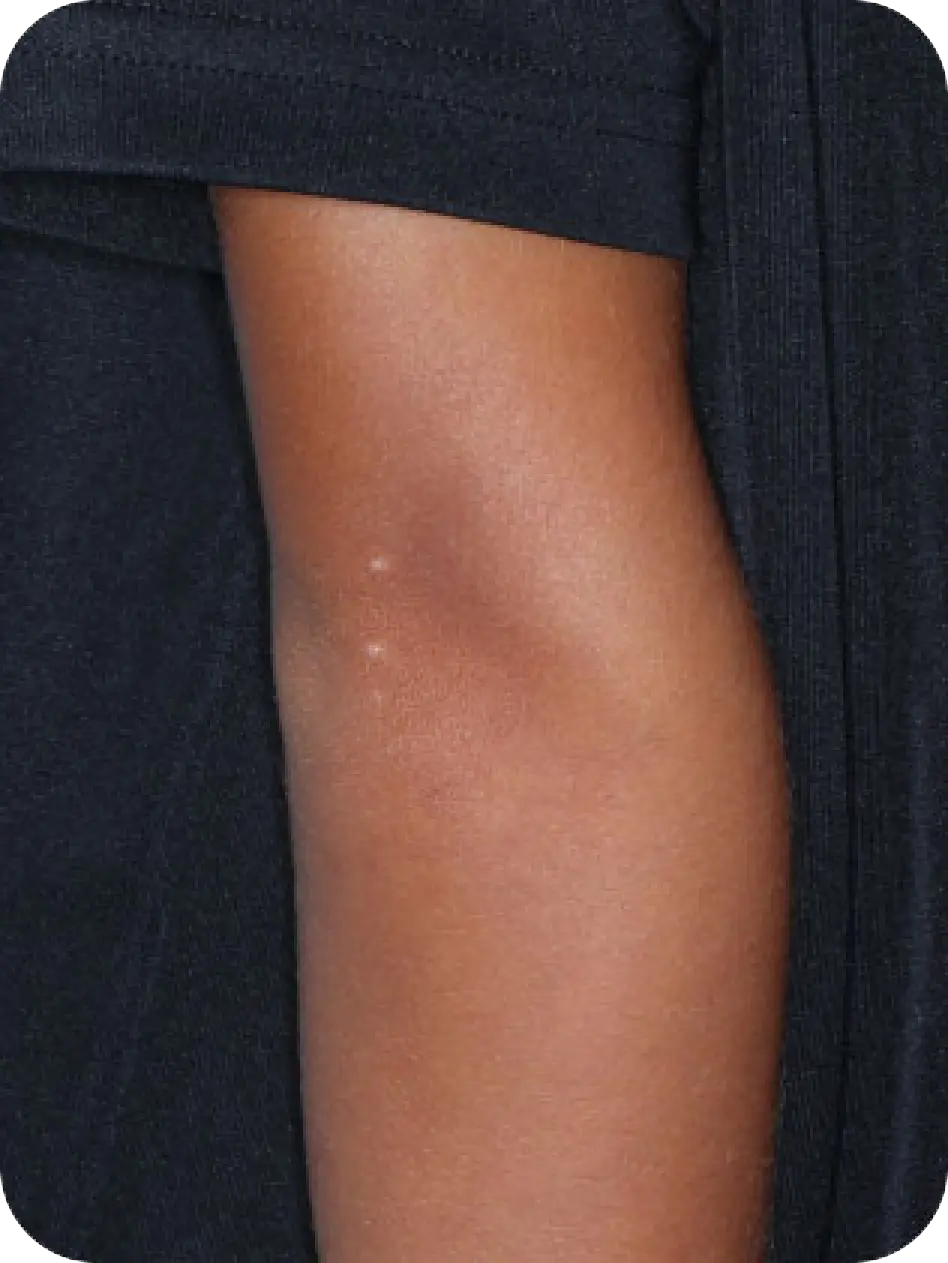
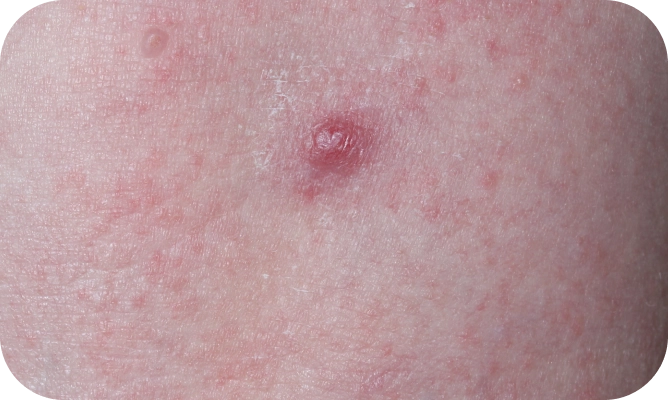
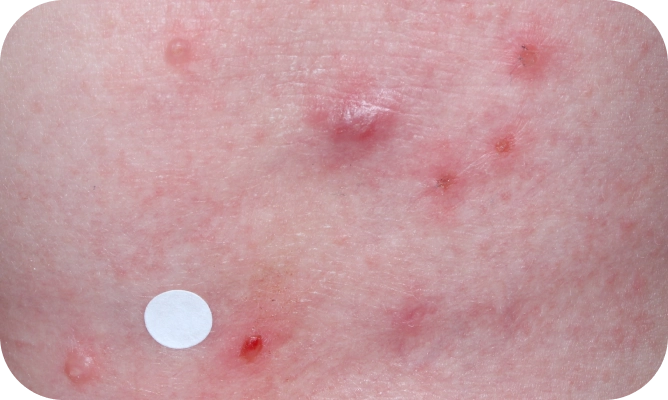
YCANTH (cantharidin) topical solution, 0.7% is indicated for the topical treatment of molluscum contagiosum in adult and pediatric patients 2 years of age and older.
IMPORTANT SAFETY INFORMATION:
What warnings should I know about YCANTH?
-
Do not get YCANTH in the mouth, nose, or eyes, and do not let YCANTH touch healthy skin. Life threatening or fatal toxicities can occur if YCANTH is taken by mouth. Avoid contact with areas of the body that have been treated, including contact by mouth. Damage to the eyes can occur if YCANTH comes in contact with the eyes. If YCANTH gets in the eyes, rinse eyes with water for at least 15 minutes.
-
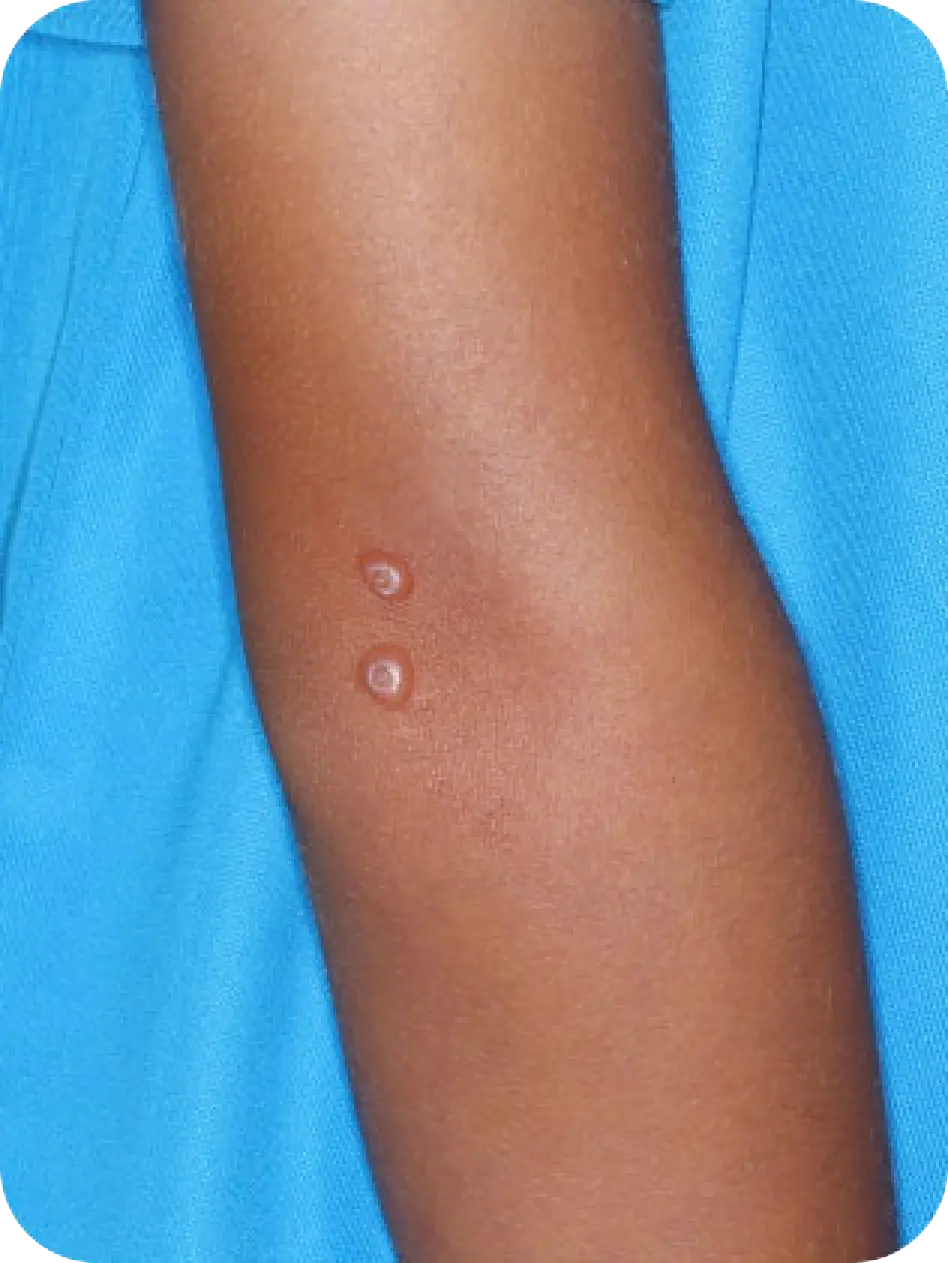
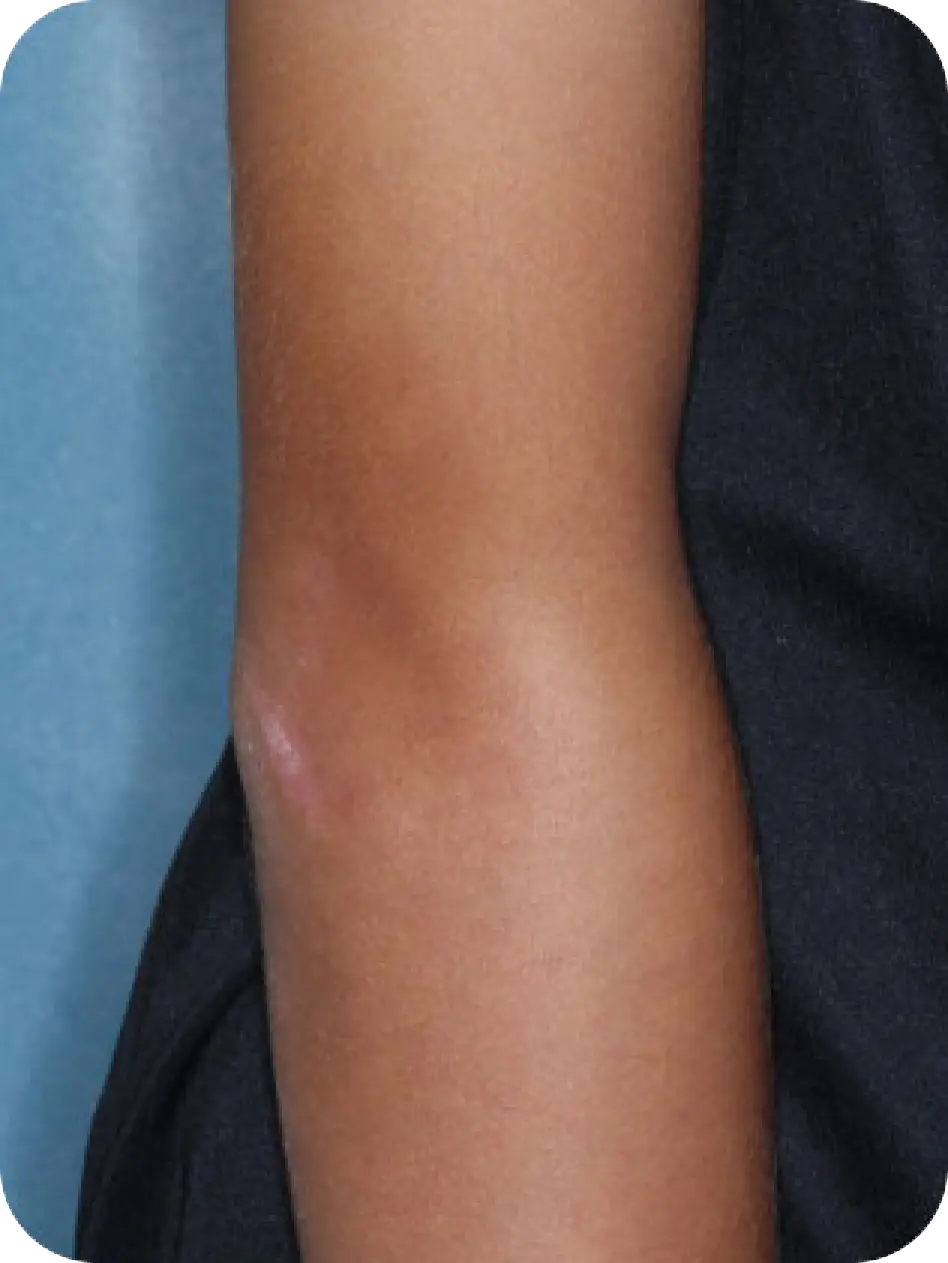
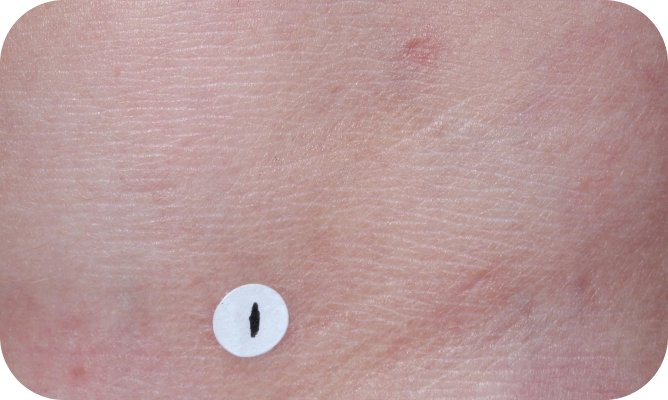
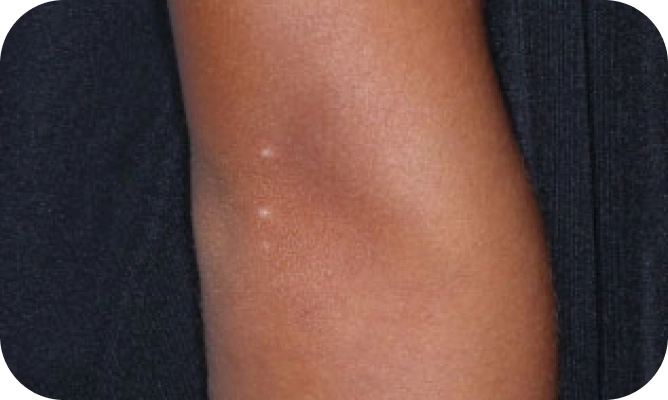
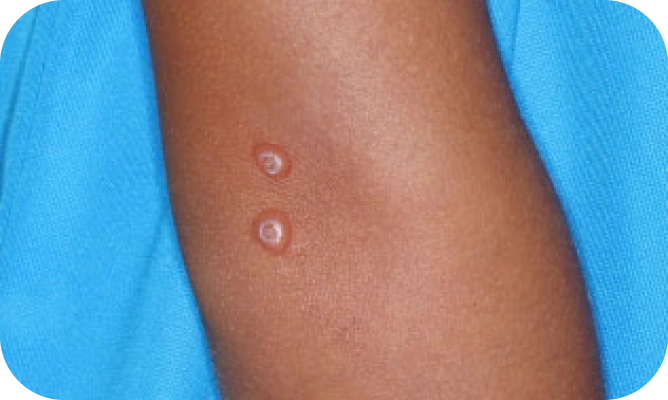
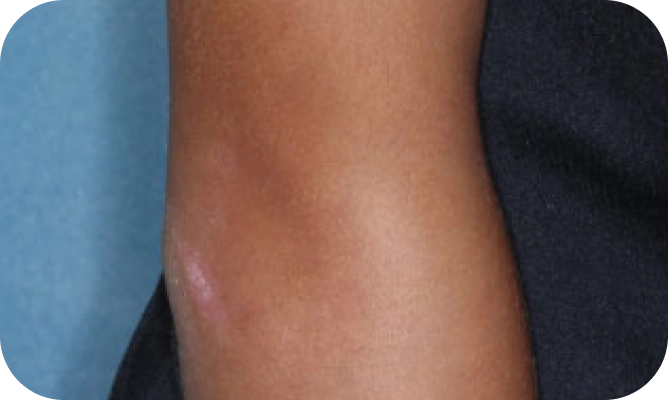
Local skin side effects at the application site may occur, including blistering, itching, pain, discoloration, and redness. Do not get YCANTH in the mouth, nose, or eyes, and do not let YCANTH touch healthy skin. If YCANTH contacts any unintended surface or healthy skin, immediately remove. If severe blistering, severe pain, or other severe skin side effects occur, wash off YCANTH immediately and contact your healthcare provider.
-
YCANTH is flammable, even after drying. Do not expose YCANTH-treated areas to fire, flame, or smoke until YCANTH is washed off.
-
Do not use YCANTH if you are allergic to any of the ingredients in YCANTH. The active ingredient in YCANTH is cantharidin. The inactive ingredients are acetone, camphor, castor oil, denatonium benzoate, ethanol, gentian violet, hydroxypropyl cellulose, and nitrocellulose.
What are the possible side effects of YCANTH?
The most common side effects are local skin side effects at the application site, including blistering, pain, itching, scabbing, redness, discoloration, dryness, swelling, and loss of the outer layer of skin at the application site. These local skin side effects are expected and are related to the anticipated blistering caused by YCANTH.
Do other medications interact with YCANTH?
It is not currently known if any medications interact with YCANTH.
Can I use YCANTH if I’m pregnant?
The potential risk of YCANTH for major birth defects, miscarriage, or maternal or fetal side effects is unknown. Given that YCANTH is applied to the outside of the mother’s skin, use is not expected to result in exposure of YCANTH to unborn babies.
Can I use YCANTH if I’m breastfeeding?
Do not use YCANTH on areas of the mother’s body where YCANTH may come in contact with the breastfeeding child’s mouth or eyes.
What should I do if YCANTH is swallowed?
Taking cantharidin by mouth has resulted in kidney failure, blistering and severe damage to the stomach and intestines, excessive bleeding or clotting, seizures, and weakness or paralysis. Seek medical attention immediately if YCANTH is accidentally ingested.
These are not all the possible side effects of YCANTH. Call your doctor for medical advice about side effects. You are encouraged to report side effects to the FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch. You may also report side effects to Verrica Pharmaceuticals Inc. at 1-877-VERRICA (1-877-837-7422). Local skin side effects are expected and should be reported if they are severe.
Please see full Prescribing Information.